
Most chemical safety testing for the eye is currently performed using the Draize method (Draize 1944), as modified by Kay and Calandra (Kay & Calandra 1962). This now-controversial procedure involves instilling the substance under evaluation within the conjunctival sac of a New Zealand White Rabbit. Indices of toxicity are recorded for the cornea, iris, and conjunctiva at regular time intervals up to 21 days. Modern chemical classification systems, including the European Union (EU), Global Harmonized System (GHS) and the Environmental Protection Agency (EPA) classification systems use the Draize test data with different analytical criteria to classify the irritation potential of materials. The EU Dangerous Substance Directive (DSD) classification and labeling system does not include cosmetics; it is applied in accordance with Commission Directive 2001/59/EC and includes categories R41 (risk of serious damage to eyes) and R36 (ocular irritant) (EC 2001). The DSD was replaced by the classification, labeling, and packaging (CLP) regulation aligned with GHS (CLP, Regulation EC No. 1272/2008). The GHS includes the classes NC (which should not be confused with the layperson’s definition of an ocular nonirritant), 2B, 2A (reversible irritation), and 1 (irreversible effects on eyes), with the score driven by 67% of all animals tested, as shown in Table 1. This lowest level of classification (NC) can include a considerable amount of damage to the eyes as long as the 3-day averages are below specific criteria.
Criteria for Classification of Irritation According to the GHS Classification System.
| GHS Category | Rabbit Category Necessary for Classification |
|---|---|
| 1 | Group A:
|
| 2A |
Rabbit with mean scores (rabbit values are averaged across observation days 1, 2, and 3) for one or more of the following:
|
| 2B |
Rabbit with mean scores (rabbit values are averaged across observation days 1, 2, and 3) for one or more of the following:
|
| Not Classified | Rabbit mean scores that fall below threshold values for Category 1, 2A, and 2B |
Table 1. Adapted from Background Review Document of an In Vitro Approach for EPA Toxicity Labeling of Anti-Microbial Cleaning Products (Curren et al.).
*Full reversal of the effects was defined as corneal, iritis, redness, and chemosis = 0.
The EPA classification, shown in Table 2, includes classes I (corrosive), II (moderate irritant), III (mild irritant, includes lesions that persist for 24 hours), and IV (no significant damage 24 hours after exposure, but may include adverse ocular effects that occur prior to 24 hours but then clear below an acceptable limitation by 24 hours), with the classification driven by the single most severe animal response and in accordance with the guidelines in the Label Review Manual (US EPA 2003), which is based on test methods described in the Acute Eye Irritation Health Effects Test Guideline (US EPA 1998).
Table 2. EPA Eye Irritation Toxicity Categories.
| EPA Category | Draize Eye Test Scoring |
|---|---|
| I | Corrosive, corneal involvement or irritation (iris or cornea score ≥ 1 or redness or chemosis ≥ 2) persisting more than 21 days or corneal effects that are not expected to reverse by 21 days |
| II | Corneal involvement or irritation clearing in 8–21 days |
| III | Corneal involvement or irritation clearing in 7 days or less |
| IV | Effects clearing in fewer than 24 hours* |
Table 2. Adapted from Background Review Document of an In Vitro Approach for EPA Toxicity Labeling of Anti-Microbial Cleaning Products (Curren et al.).
*Based on positive scores for conjunctival irritation ≥ 2.
Modern EPA and GHS ocular classification systems have replaced older score-based indices of ocular irritation [chiefly, the “Modified Maximum Average Score (MMAS) represents maxima calculated at 24 hours or more following instillation] (ECETOC Technical Report 1998). Because it would be inhumane to test the large number of chemicals required for nonanimal test validation, several retrospective databases (for example, ECETOC Technical Report 1998 and Barroso et al. 2017) can be used to calculate the EPA and GHS classifications. The important advantage to using retrospective in vivo databases is that no more animal testing would be performed; however, it should be noted that these databases are very old, with mostly single studies with three or sometimes six animals each, and there is a high variability between animals, for example, see ECETOC (ECETOC Technical Report 1998) and, in the few cases of multiple studies, a high between-study variability (Barroso et al. 2017).
The high variability of the Draize eye test and the limited number of classification drivers result in misclassification errors by both the GHS and EPA classification systems (Barroso et al. 2017). “Resampling has demonstrated an overall probability of at least 11% that chemicals classified as Cat. 1 by the Draize eye test could be equally identified as Cat. 2 and about 12% of Cat. 2 chemicals could be equally identified as NC.” (Adriaens et al. 2014). Cosmetics Europe suggested that about 73% of the 511 chemicals tested in the Draize eye test Reference Database (DRD), including drivers of classification, are considered good reference chemicals; reasons for exclusion include chemicals that were tested in a single animal but showed severe and persistent effects that were classifiable as Cat. 1 and chemicals identified as Cat. 2A or 2B in repeated studies (Barroso et al. 2017). Therefore, validation studies using these data may not be optimally evaluated due to the inconsistency of the gold standard to which they are compared.
The OptiSafe Test Method
In 2017 the test method was validated in collaboration with NICEATM, ICCVAM and the NTP. The NTP provided coded vials of the selected test chemicals to the lead lab and two naïve laboratories. Interlaboratory reproducibility, accuracy, and false-negative rates were 91%, 89%, and 0%, respectively, for both the EPA and GHS classification systems. The false-positive rates were 23% for the GHS classification system and 25% for the EPA classification system. The success of these accuracy and transferability studies are further emphasized by the findings that there was low variation in irritation scores with similar final classifications for EPA Cat. IV versus the rest or GHS not classified (NC) versus the rest with transferability greater than 90%. This finding indicates that the OptiSafe test method is reproducible by naïve laboratories and demonstrated reliability in manufacturing processes with little or no lot-to-lot variability, as shown by the low variation of irritation scores and the lack of any statistically different irritation scores between three different lots of OptiSafe test kits.
Overall, the OptiSafe method performed better than all other test methods to which it was compared with differences in performance attributable to the superior sensitivity of the OptiSafe test method. This study’s data are consistent with the initial prevalidation data submitted to NICEATM and reviewed by ICCVAM, in which it was concluded that the OptiSafe test method compares favorably with other nonanimal test methods.
The OptiSafe test method allows users to start with any unknown test substance, including unground solids and intensely colored substances, etc., and determine the irritancy classification based on specific biochemical assays. To ensure the highest accuracy and streamline testing, pretests are performed. Pretests are applied to a decision tree that indicates which main test procedures should be performed.
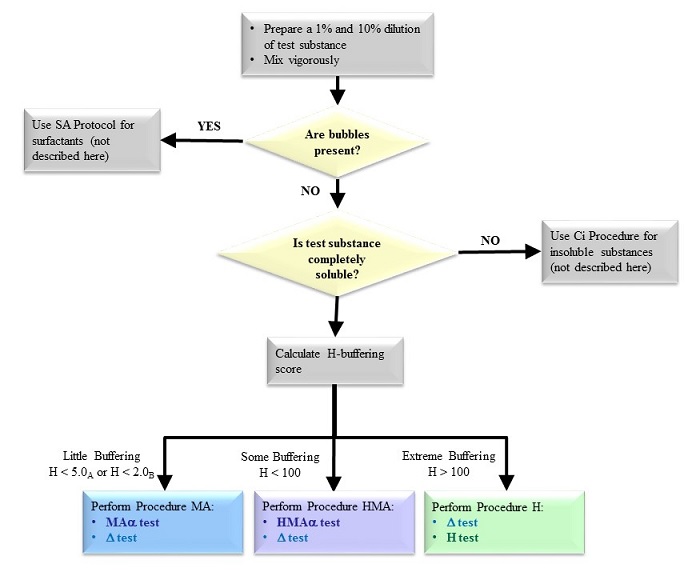
Figure - illustrates the pretest flowchart. The prescreening procedure is utilized to differentiate test substances and assign the primary assay that corresponds to the chemical and physical properties of the test substance. The main assays are inclusive to many chemicals compounds including surfactants, nonsurfactants, and insoluble substances.
Main Test Procedures
- Procedure α – assess collagen denaturation and oxidative damage/excessive reactivity
1. MAα – nonsurfactants with limited or little buffering capacity
2. HMAα – nonsurfactants with moderate buffering capacity
3. Ci – insoluble compounds
- Procedure Δ – assess water insoluble denaturation and oxidative damage/excessive reactivity
- Procedure Η – assess ocular pH shift
Figure 2 shows an overview of the alpha procedure. This test method models denaturation and damage to biological molecules and tissues. The active agent is aliquoted into a 24-well plate, discs are added to each experimental well, and the test substance and standard controls are titrated into the wells of the ocular membrane discs. After 18 hours of incubation, the results are quantified using optical density values from a spectrophotometer. The increase in optical density value indicates an increase in damage, representative of denaturation and reactivity of the test substance with the active agent. Finally, an irritancy classification is assigned to the compound.
Figure 2. Alpha Test Flow Chart.
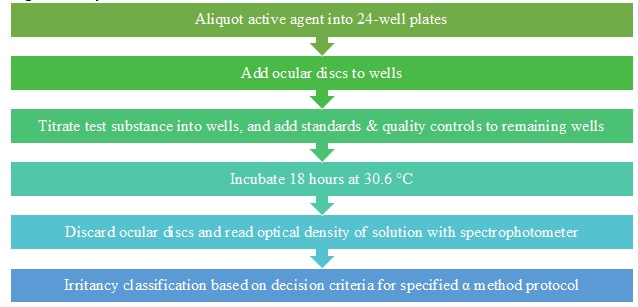
Figure 3 shows the delta procedure. This test method is applicable for water insoluble or slightly soluble substances to assess damage to membranes, and oxidative and excessive reactivity. The test sample and standard controls are aliquoted to individual incubation cells, and a delta reagent is added to each respective cell. Following 18 hours of incubation, the results are read using a spectrophotometer to determine optical density and then an irritancy classification is assigned.
Figure 3. Delta Test Flow Chart.

Figure 4 shows the H procedure. This method is used to evaluate the ability of a test substance to shift ocular pH significantly; extreme variations of pH are detrimental to ocular tissue function. A buffered solution is adjusted to a pH of 7.4 and added to the titrated test substances, ranging from 2% to 10% concentration. Once the solution is vortexed, the solution is left to incubate for 10 to 15 minutes, and the resulting pH is measured. Based on the test substance’s capability to shift the pH, an H score is calculated, and an irritancy classification is determined.
Figure 4. Η Test Flow Chart.

Scored indices of eye toxicity result from both direct lesions and later host system responses to the initial molecular disruption caused by the substance under study. Direct lesions result when chemicals react with or otherwise disrupt the molecules that make up the tissues of the eye. For example, disruption of the corneal epithelium through the lysis of plasma membranes leads to pain and corneal swelling, whereas deeper injury to the eye through protein denaturation leading to corneal stromal collagen disorganization results in further opacity and loss of tissue function. These early events are then followed by further indirect disruption of ocular function through graded neurogenic and immune system responses to these molecular disturbances, leading to compensatory inflammatory cascades and release of cytokines that further cause tissue swelling, pain, redness, increased temperature, and further destruction of the ocular tissues. Since past studies have shown that these graded indirect responses that ultimately determine the reversibility or irreversibility of the ocular damage are directly dependent on the severity or extent of the initial direct damage, it is theoretically possible to link the ability of a material to cause direct damage to the final outcome of the ocular irritation response and therefore predict the level of ocular irritation caused by the material. Specifically, by modeling mechanisms of tissue injury and measuring the graded potential of a material to cause acute tissue disruption, one may be able to predict the final outcome and resolution of the ocular irritation response without measuring the indirect neurogenic and immune tissue responses that lead to the final outcome of the ocular irritation. Potentially, these measurements should not require use of ocular tissues, but only modeling of specific biochemical and biophysical interactions that underlie the initial ocular tissue disruption.
Evaluated Mechanism of Injury
Different ocular irritants, including acids/bases, alcohols/aldehydes/ketones, strong oxidants, and reactive chemicals/mixtures, damage the cornea and other tissues through additive and/or synergistic chemical reactions and biophysical effects. Evaluated mechanisms of injury include the following:
- Denaturation of specific water-insoluble polymers that model the phospholipid bilayer of cells (which can occur in the corneal epithelium and conjunctiva)
- Direct denaturation of macromolecules that model ordered collagen (which can occur in the corneal stroma)
- Indirect denaturation of molecules across a membrane via osmotic effects (which can occur across the corneal epithelium and stroma to damage the cornea)
- Damage to tissues via excessive oxidation and reactivity (which can occur in the epithelium, stroma, conjunctiva, and iris)
- Damage to tissues via extreme buffering (which can occur in the epithelium, stroma, conjunctiva, and iris)
SERVICES AND KITS
OptiSafe cosmetic and personal care product testing services are available from
BioScreen Testing Services
Phone: (310) 214-0043
http://www.bioscreen.com/index.php/forms-a-quotes
Kits are available from
Lebrun Labs
Phone (714) 345-4689
sales@lebrunlabs.com
Research reported in this publication was supported by the National Institute Of Environmental Health Sciences of the National Institutes of Health under Award Number R44ES025501. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health